Maldives approves Pfizer, Sinopharm vaccines for emergency use
The Madlives has authorized two COVID-19 vaccines for emergency use
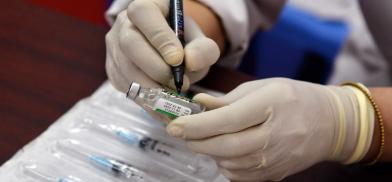
The Madlives has authorized two COVID-19 vaccines for emergency use. The Maldives Food and Drug Authority (MFDA) has given approval to China’s Sinopharm vaccine and the Pfizer-BioNtech vaccine.
The Health Emergency Operation Centre (HEOC)’s Spokesperson, Dr. Fathmath Nazla Rafeeq said the Sinopharm vaccine has been approved by Maldives Food and Drug Administration (MFDA) to be administered to those between 18 and 60 years of age.
The Sinopharm vaccine has not yet been approved by European regulators. Therefore, several aspects were reviewed regarding the vaccine, Dr. Nazla was quoted by avas.mv.
This includes manufacturer information and other data from countries that have already granted approval for the vaccine.
The Maldives has previously used Indian-made Covishield vaccines. The island nation has reported 64 deaths and over 21, 000 COVID-19 cases.





Post a Comment