Nepal approves India's Covishield vaccine for emergency use
Ahead of signing a major Covid vaccine deal with India, Nepal's Department of Drug Administration (DDA) has given permission for the emergency use of India-made coronavirus vaccine Covishield in the country
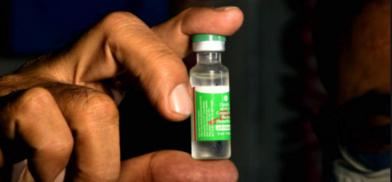
Ahead of signing a major Covid vaccine deal with India, Nepal's Department of Drug Administration (DDA) has given permission for the emergency use of India-made coronavirus vaccine Covishield in the country.
The vaccine, developed by the Oxford University and Astra-Zeneca and produced by the Serum Institute of India, has been given conditional permission for emergency use, the department said.
The DDA had two days ago called for the registration of vaccines to be used for emergency use.
This is the first time that any Covid-19 vaccine has been approved for use in Nepal. Other manufacturing countries like China and Russia were also interested in providing vaccines to Nepal but due to climatic conditions, pricing, logistic facility and ease of import, Nepal preferred the Indian Covid vaccine.
India has already permitted the use of Covishield as well as Covaxin of Bharat Biotech.
(IANS)




Post a Comment